How Pandemics Changed The Way We Develop and Apply Point of Care Diagnostics
In Vitro Diagnostics (IVD) may be a small part of overall healthcare costs, but they influence two-thirds of all healthcare decisions with huge implications for patients with diabetes, heart disease, cancer, respiratory ailments, and more.
What we learned from the pandemic
If there is a bright side it’s the fact that our eyes are now wide open to the importance of rapid, accurate detection of diseases and viruses.
Point of care testing (POCT) technology has been around for decades but has really become much more sophisticated in the last 10 years thanks to advancements in microfluidics and biosensing technologies. These technologies, also called Lab On a Chip (LOC) systems, now perform tests that used to be done by far more sophisticated and expensive diagnostic lab systems. This revolution is being driven by micro and nanotechnologies for the miniaturization of onboard circuitry, vast improvements in fluid handling and biosensor technologies, and the arrival of industrial technology leaders to the global Dx supply chains. These advances have converged to allow us to make point of care tests smaller, more accurate and more cost competitive than ever before.
Getting the right result, right now, is more important than ever.
- Francisco Blanco Barro,
- Global Head of Commercial and Strategy TE Connectivity – IVD Solutions Team
Point Of Care Tests: Front and Center
Most people were familiar with blood and urine tests before the pandemic but have little or no understanding of the underlying technology. Beyond blood glucose, home pregnancy and genetic tests, few people had administered a rapid Point of Care home IVD test. COVID blew that wide open. Billions of people worldwide have now had at least one rapid COVID antigen or PCR test, administered at home or by a medical professional. The results have been staggering. People are now diagnosed with COVID in 15-30 minutes, allowing them to immediately isolate and get treated, or get back to their normal routine.
Expansion of portable rapid testing equipment offers enormous benefits in underserved and rural areas of the world where travel may be a barrier for patients to seek healthcare or return the following day for test results and treatment.

Point of care test offers enormous beneficts
Urgent Problems Produce Big Solutions
The international effort to sequence the 3 billion DNA letters in the human genome is considered by many to be one of the most ambitious scientific undertakings of all time, even compared to splitting the atom or going to the moon.
Launched in 1990, the Human Genome Project (HGP) was an international effort focused on determining the base pairs that make up human DNA and then sequencing all of our genes. Most scientists would agree HGP was a huge leap forward in our understanding of the genome and vastly accelerated the development of new diagnostic approaches - a major advancement for humanity. The development of the SARS-CoV-2 (COVID) vaccines should be viewed as a similarly remarkable achievement thanks to the focus of governments and companies to tackle this enormous challenge, along with a multitude of impressive tests that followed.
This rapid response was made possible by many teams across the world working together and previous fundamental RNA research done in the wake of the SARS coronavirus epidemic between 2002-2004. This global effort most certainly saved hundreds of thousands of lives worldwide. TE Connectivity (“TE”) played a role by helping customers at diagnostic companies compress test development timelines. Regulators, usually unmotivated by speed, should also be credited for quickly issuing Emergency Use Authorizations for rapid tests, allowing many people to resume their normal life and speeding economic recovery.
The Testing Revolution Is Just Getting Started
We are now on the cusp of the next revolution in diagnosis, driven by advances in microfluidic LOC technology and the confluence of:
- Ever more powerful, smaller and competitive microfluidic chips and biosensors
- Smart interconnected instruments and readers to perform portable benchtop or even handheld analysis
- Sophisticated Design for Manufacturing (DFM) techniques that allow for a seamless and swift transfer from prototype to full scale manufacturing
- Global manufacturing that allows for less expensive and more adaptive assembly
TE works with IVD companies to design and develop new POCT cartridges based on the most advanced microfluidic and biosensor technologies, tests them in-house, and then seamlessly transitions the design from limited-scale runs to large scale manufacturing at various TE facilities globally. This type of manufacturing flexibility helps companies scale more quickly and keep costs low.
More Sophisticated Point of Care Tests Herald Emerging Applications for Microfluidic Technology
COVID brought significant advances in microfluidic technology and what we learned has the potential to accelerate the trend of personalized medicine. Microtechnology and novel biosensor devices allow microfluidic devices to manipulate tiny amounts of fluid, making them appropriate for point of care applications where an accurate result is needed right now using a simple portable format. Additional respiratory, gastrointestinal and oncology applications will emerge using microfluidic technology, replacing some expensive and slower benchtop systems in the process.
The Future Of POCT: Lab Quality Results in Under 30 Minutes
You may be able to relate to this scenario: you go to your doctor, they take a sample and send you on your way, perhaps with a prescription you may (or may not) need. You wait…and wait. Finally, your doctor’s office calls back days later with your test results, and you may need to go back to the doctor’s office to find out what’s next. This game plays out across the world millions of times each day and it’s incredibly inefficient.
Now that most consumers are familiar with rapid tests, expectations have changed. Consumers expect to know the results of tests faster, and doctors would prefer to correctly diagnose patients while they are in their offices the first time. This technology has a big role to play in lowering costs and improving outcomes because patients can be tested, diagnosed and start treatment in one visit.
Do I Have a Cold, Flu, Pneumonia or COVID?
That question was not quickly answered 5 years ago, and certainly not with one test. Respiratory infections are caused by bacterial or viral pathogens. Influenza, colds, pneumonia and allergies present similar initial symptoms that make them hard to distinguish from COVID. Yet, the severity and treatments are very different.
Flus and colds were virtually non-existent during COVID but the medical community expects them to resurface, presenting an even greater need for on-site rapid diagnosis. When these pathogens are more prevalent, patients may automatically assume they have COVID. However, they may have a cold or flu, which both can equally threaten public health but require different treatment.
Fortunately, there is now a way to distinguish between maladies that present similar symptoms. Today’s multiplex (aka: syndromic testing) molecular diagnostic tests can detect multiple pathogens at once – be it viral, bacterial, fungal or parasitic. Multiplex technology is superior to bacterial cultures and microscopy because it can simultaneously test for viruses, bacteria, yeast, parasites and antimicrobial resistant genes.
For example, numerous studies have shown that antibiotics are widely overprescribed by doctors, especially for pneumonia, cystitis, urinary tract and kidney infections. Antibiotics only work on bacterial infections and are completely useless against viruses. Because of misdiagnosis, the patient will likely end up using antibiotics for days (unsuccessfully) while their condition gets worse. Multiplex molecular diagnostic tests can eliminate the guesswork and get patients on the correct path to treatment right away.
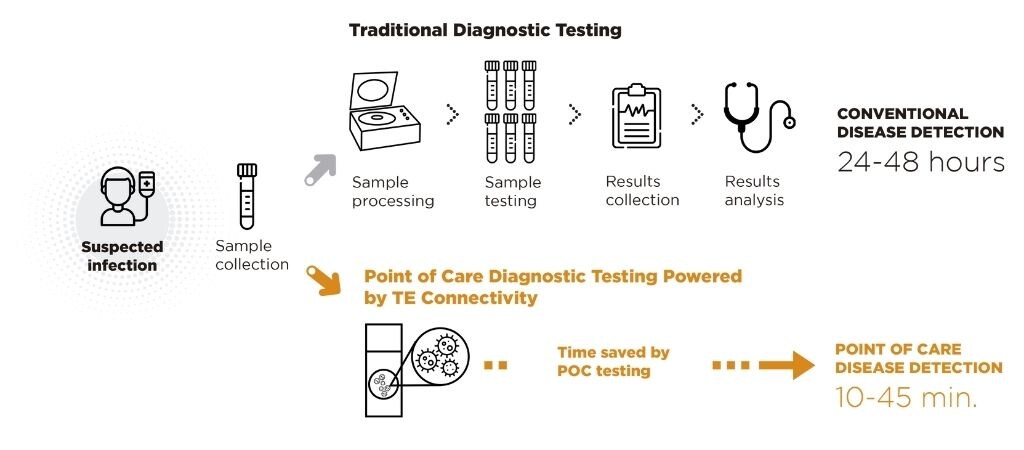
Illustration comparing typical molecular test lab process to faster POCT process
Benefits of multiplex molecular Dx tests
- Faster diagnosis
- Shorter patient stays due to correct initial diagnosis
- Binary answers – non-subjective
- Fewer or shorter duration of antibiotic use
- Better outcomes
- Lower healthcare costs
- Less throughput pressure on clinical lab staff
While large multiplex panel systems are not new, new POC devices are being designed to be smaller, faster and as accurate as their more expensive lab counterparts. This means they can be more widely applied in a variety of settings. That’s important because lab tests typically require a specialist technician to collect samples, run them and interpret results. Newer lab on a chip technologies use highly intuitive instrument or reader interfaces, simple sample loading and clear binary results guided by highly specialized usability testing. This allows these tests to be run by generalists with minimal training, opening the door to many field applications.
It’s not so hard to imagine a day when parents, urgent care, campus healthcare and nursing home staff have simple, inexpensive handheld multiplex readers with cartridges that can quickly diagnose the common cold or flu from pneumonia and other respiratory pathogens.
Advances in syndromic testing are being made possible in part using sophisticated microfluidic designs. The benefits of being able to correctly diagnose a patient using one test, during one visit, has huge implications for lowering healthcare costs and improving patient outcomes.
- Luis Fernandez,
- Global Head, New Product Development TE Connectivity – IVD Solutions Team
The Path Forward for POCT Using Microfluidic Technology
The future of Point of Care testing is exciting because the need is so great, and the benefits so immense. The computing power, ubiquity and display capabilities of smartphones (example) can extend the application of microfluidic lab on a chip devices, creating exciting new growth opportunities for testing. Giving patients and users the right diagnosis at the right time will definitely improve our healthcare system, allowing more efficient and sustainable personalized treatment.
Efficient use of reagents, more sophisticated biosensors and connections with smartphones will slowly bridge the gap in test sensitivity and specificity with more sophisticated instrument-based systems used in hospitals and labs. To reach its full potential, the next phase of POCT development will focus on lowering substrate material costs, ensuring reliability, lowering manufacturing costs and improving recyclability of disposable cartridges. TE Connectivity’s IVD Solutions team will be on the forefront of POCT design and development, helping our customers bring innovative point of care technologies to market faster than ever before.
About the Author

Fran Blanco
Fran Blanco is the global head of commercial and strategy for TE Connectivity’s in vitro diagnostics (IVD) business. He is responsible for strategy, sales and marketing of a solutions portfolio that includes new product development, regulatory, usability, clinical research and contract manufacturing services. Before joining TE, Fran worked in multiple functions and industries and held senior management roles, including chief technology officer, at global multinational companies. He has spearheaded development of new business units in the life science and energy sectors. He also co-founded two successful companies in the microfluidic and point of care (POC) fields. Fran earned his master’s degree in physics and a Ph.D. in electrical engineering in a pioneer thesis in the microfluidic POC-IVD field in the early 2000s. He completed a business-administration and innovation program at Cambridge Judge Business School (UK) focusing on spin-offs, innovation and intra/entrepreneurship. Fran has published more than 20 peer-reviewed scientific papers and is named on several patents and patent applications. He sits on several company boards of public-private organizations and high-tech start-up companies.